Timing Of Operation Warp Speed Vaccine Efficacy Trials
Timing of operation warp speed vaccine efficacy trials.
Timing of operation warp speed vaccine efficacy trials. Deputy director chief medical officer vaccine research center. It will promote mass production of multiple vaccines based on preliminary evidence allowing for faster distribution if clinical trials confirm one of the vaccines is safe and effective. Operation warp speed the federal effort to rush a vaccine to market has promised pfizer 195 billion to deliver 100 million doses to the federal government which will be given to americans. National institutes of health.
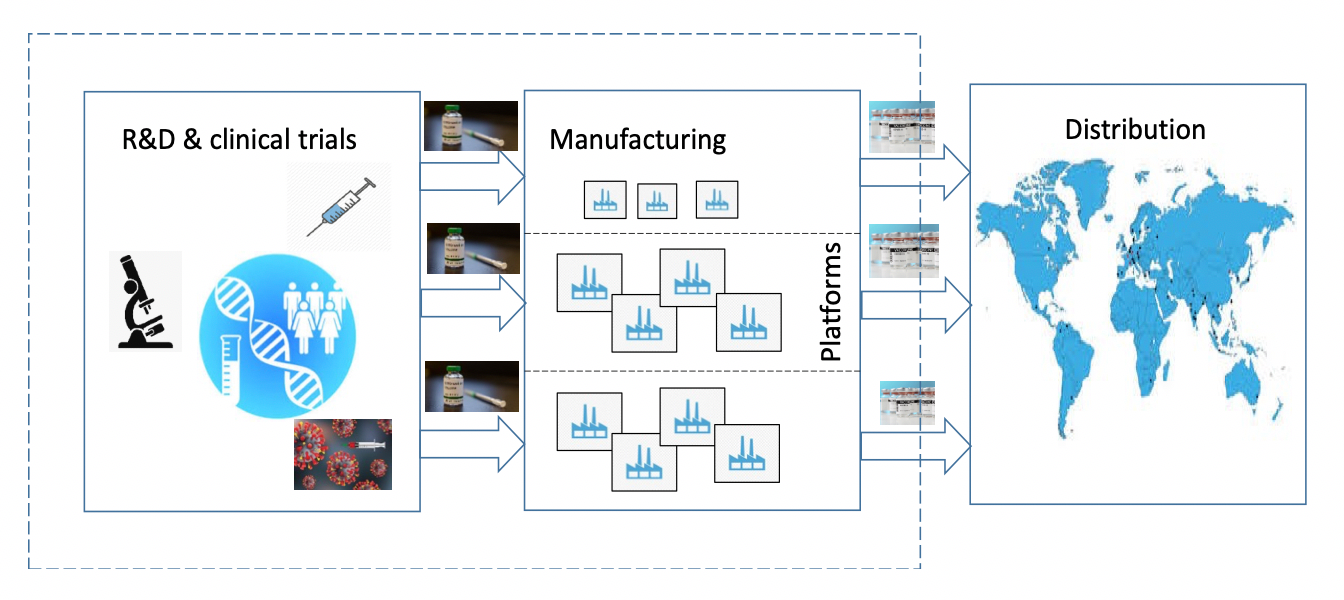
National institute of allergy and infectious diseases. Operation warp speeds goal is to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by january 2021 as part of a broader strategy to accelerate the development manufacturing and distribution of covid 19 vaccines therapeutics and diagnostics collectively known as countermeasures. Jansen sought to distance the company from operation warp speed and presidential politics noting that the company did not take any federal money to help pay for research and. Pfizer unlike its competitors did not join operation warp speed the government initiative designed to erase the financial risk of vaccine and therapeutics development by providing upfront.
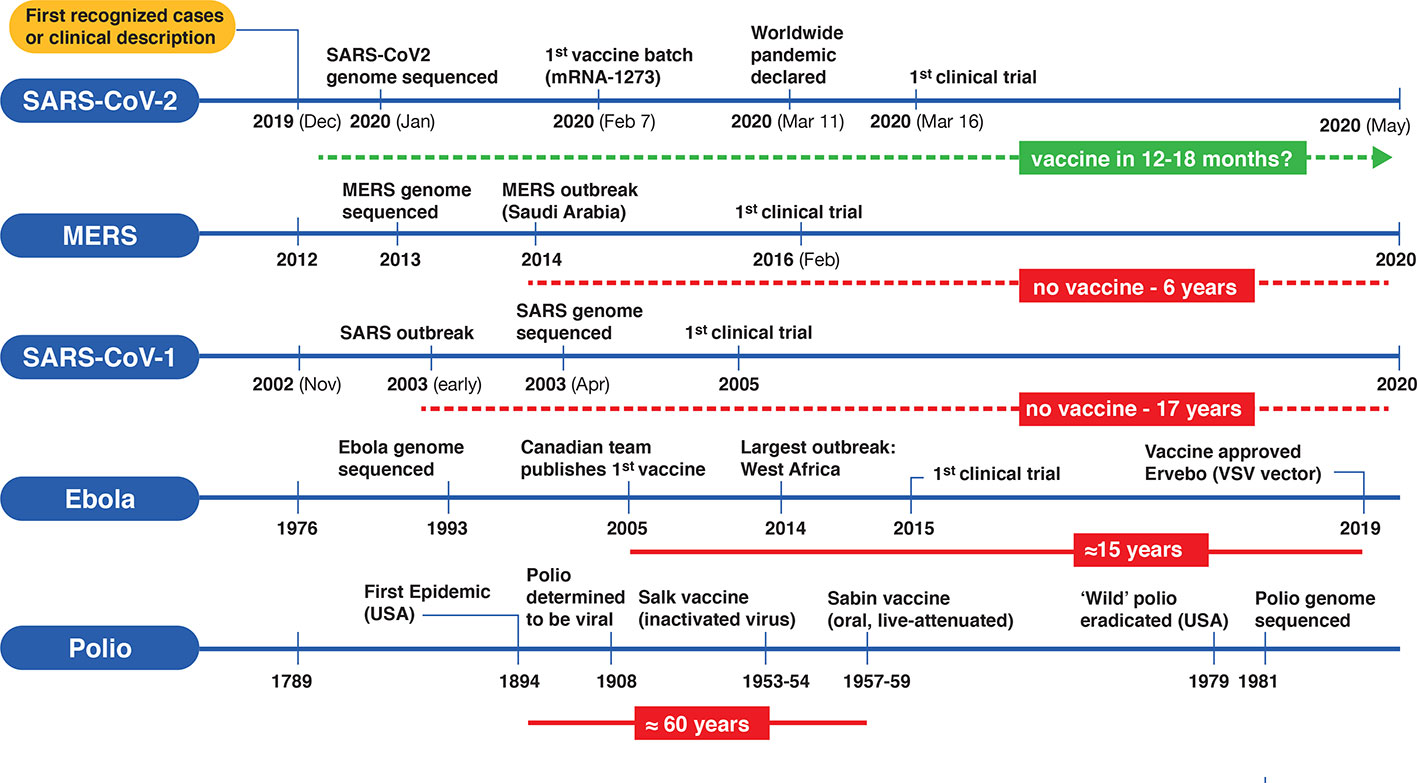
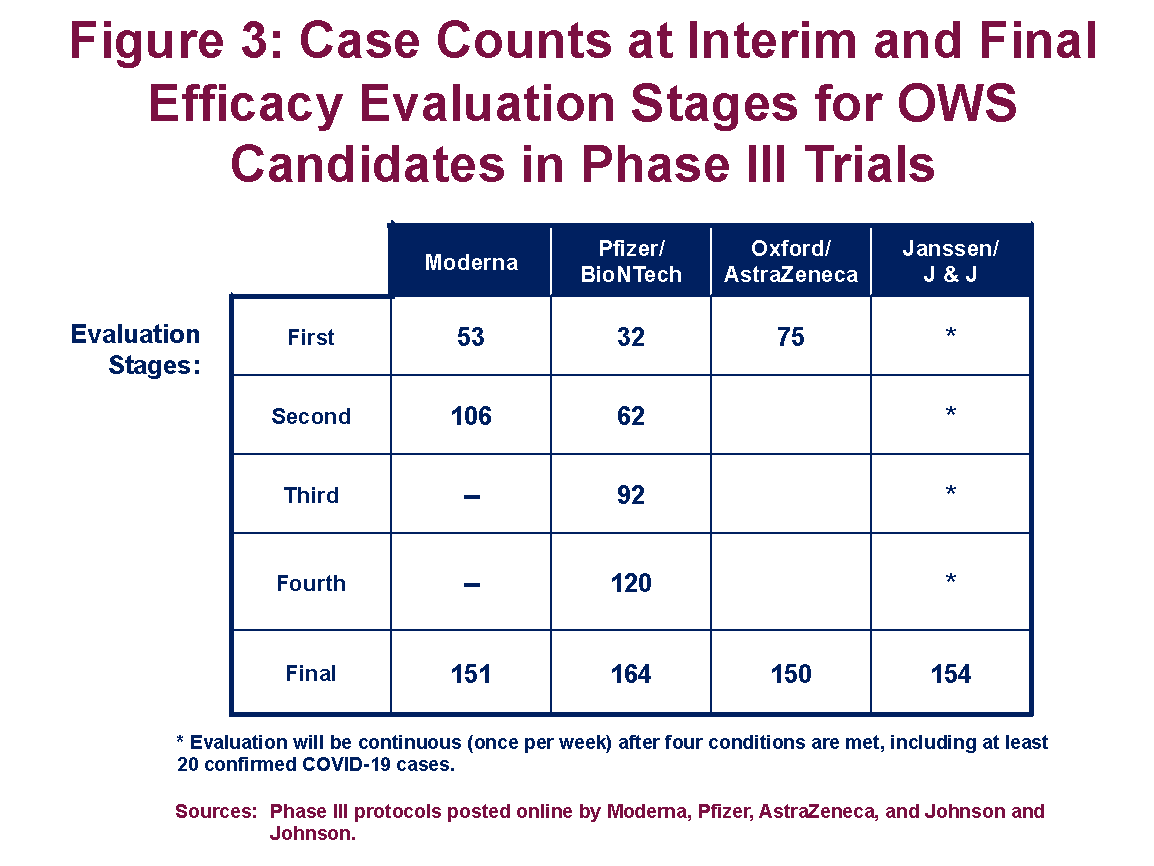
Covid 19 vaccine effort plan to take interim looks at data based on different. Coronavirus updates large scale safety and efficacy trials of experimental vaccines in tens of thousands of volunteers are underway. Operation warp speed was introduced in early april 2020 after a round table meeting with industry executives at the white house on march 2. Operation warp speeds updates covid 19 vaccine timetable.
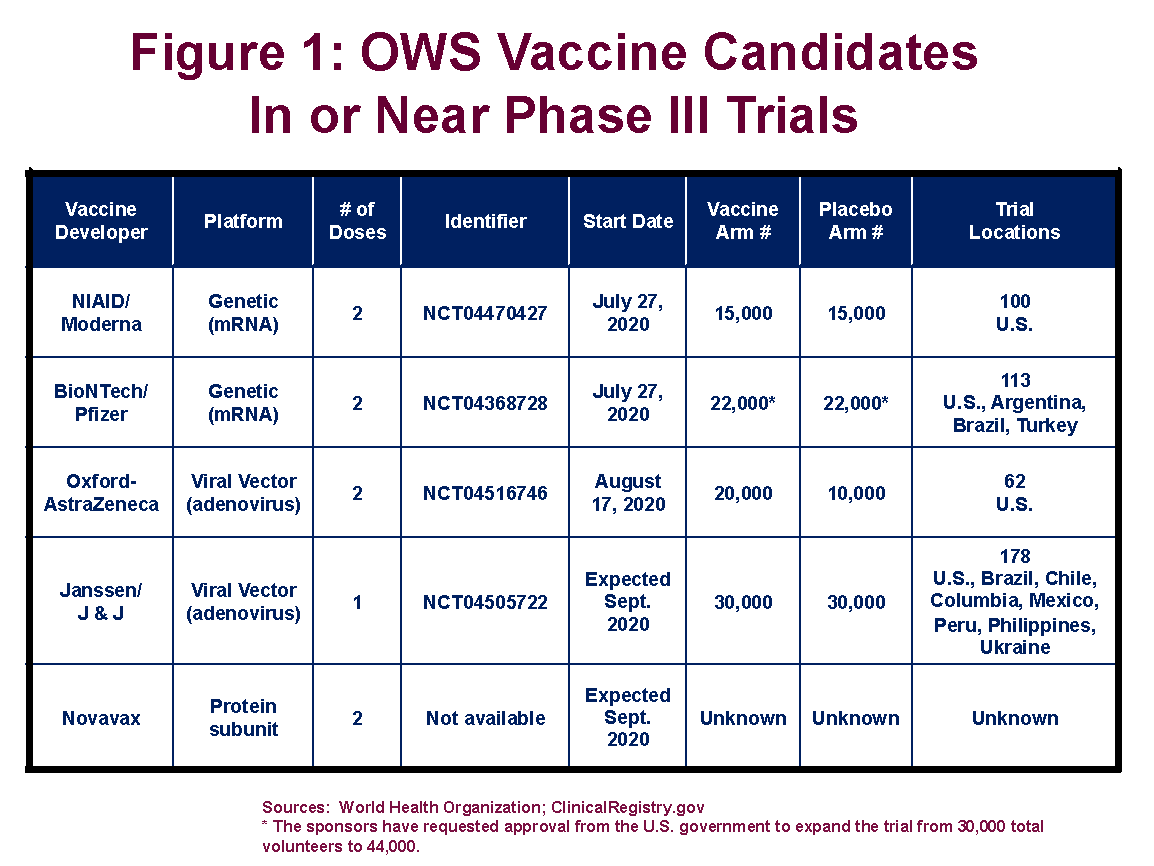
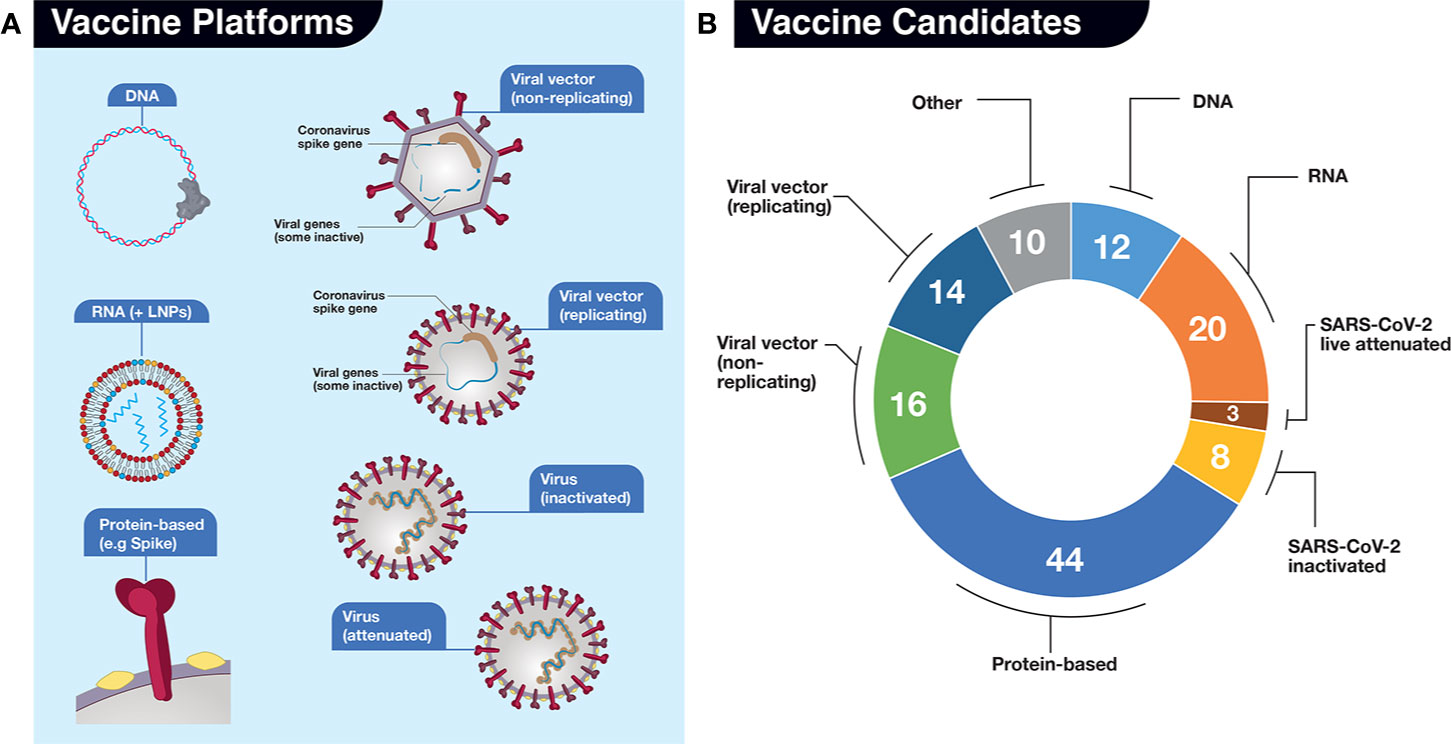
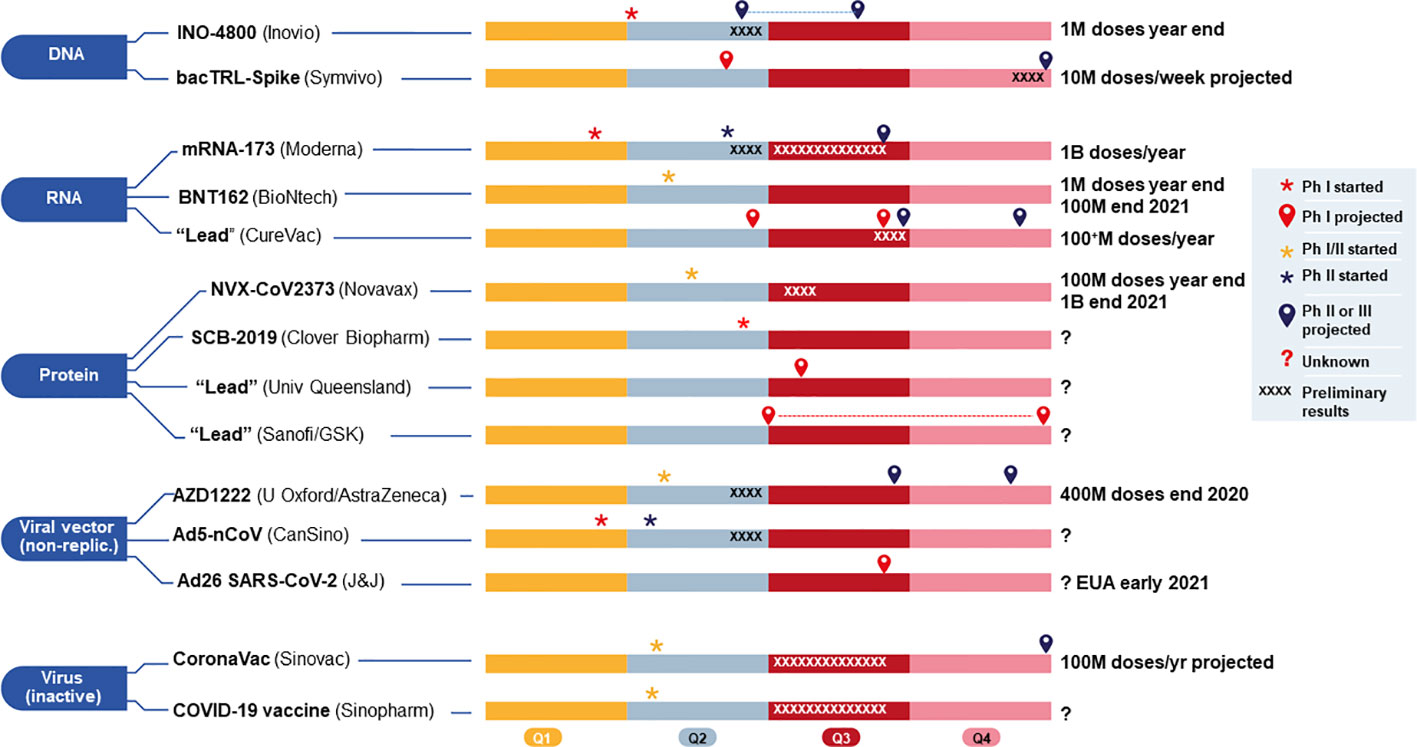
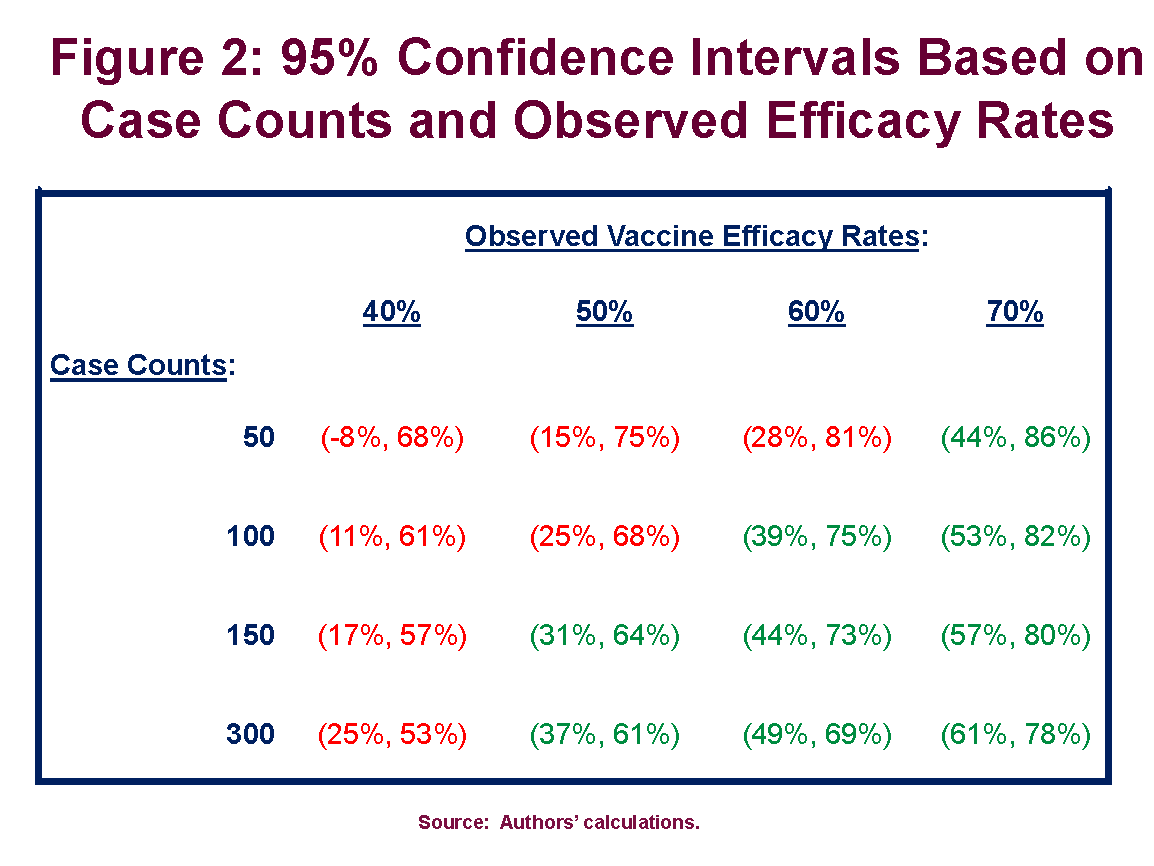
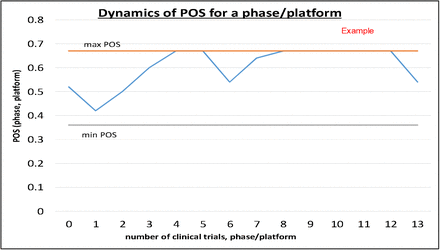
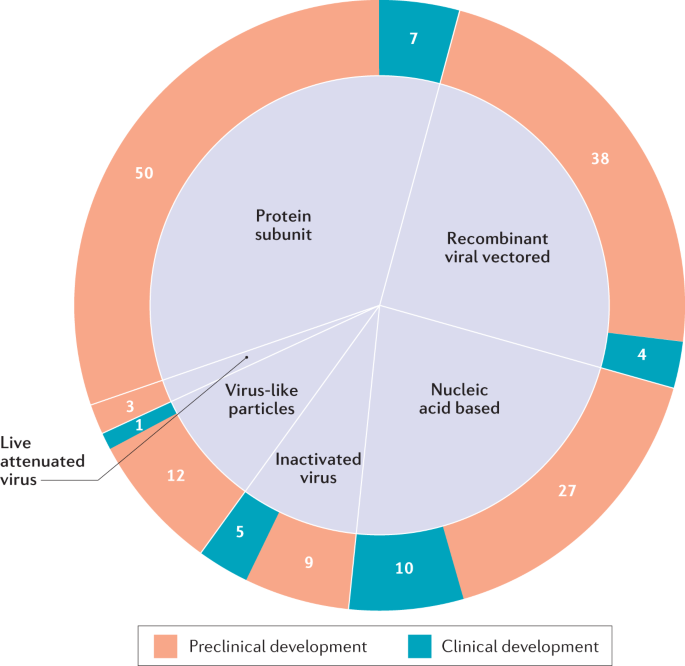
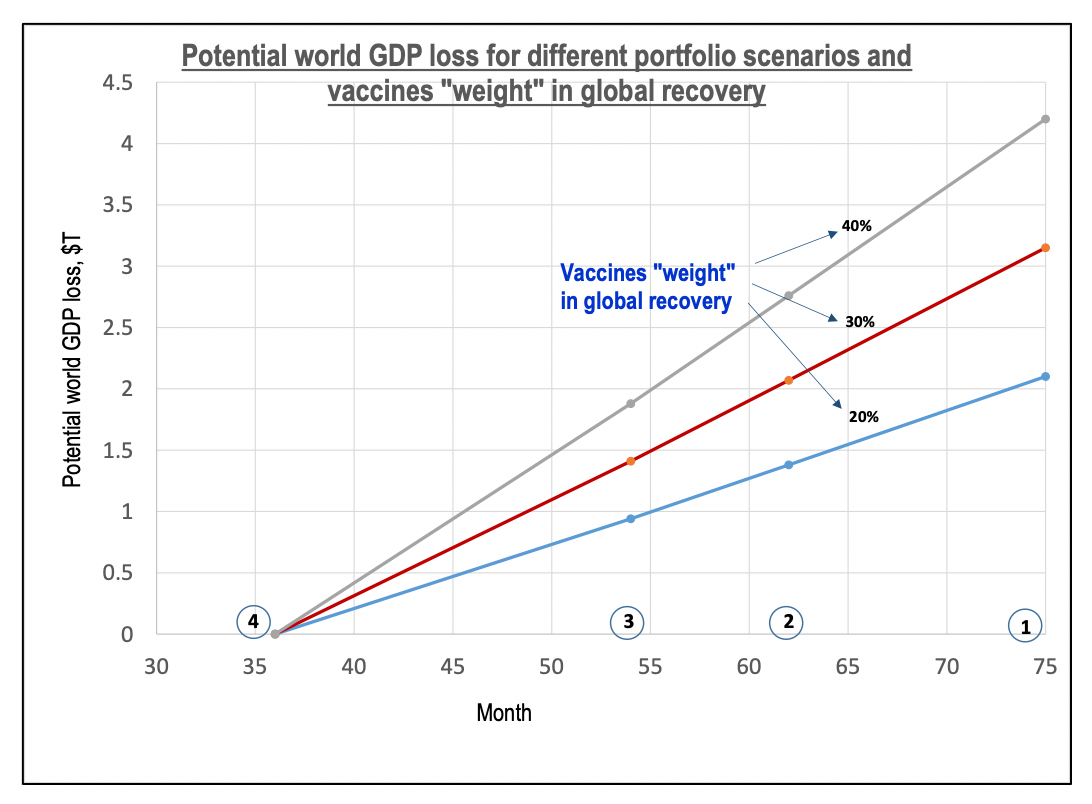
Overview of covid vaccine efficacy trials july 29 2020.










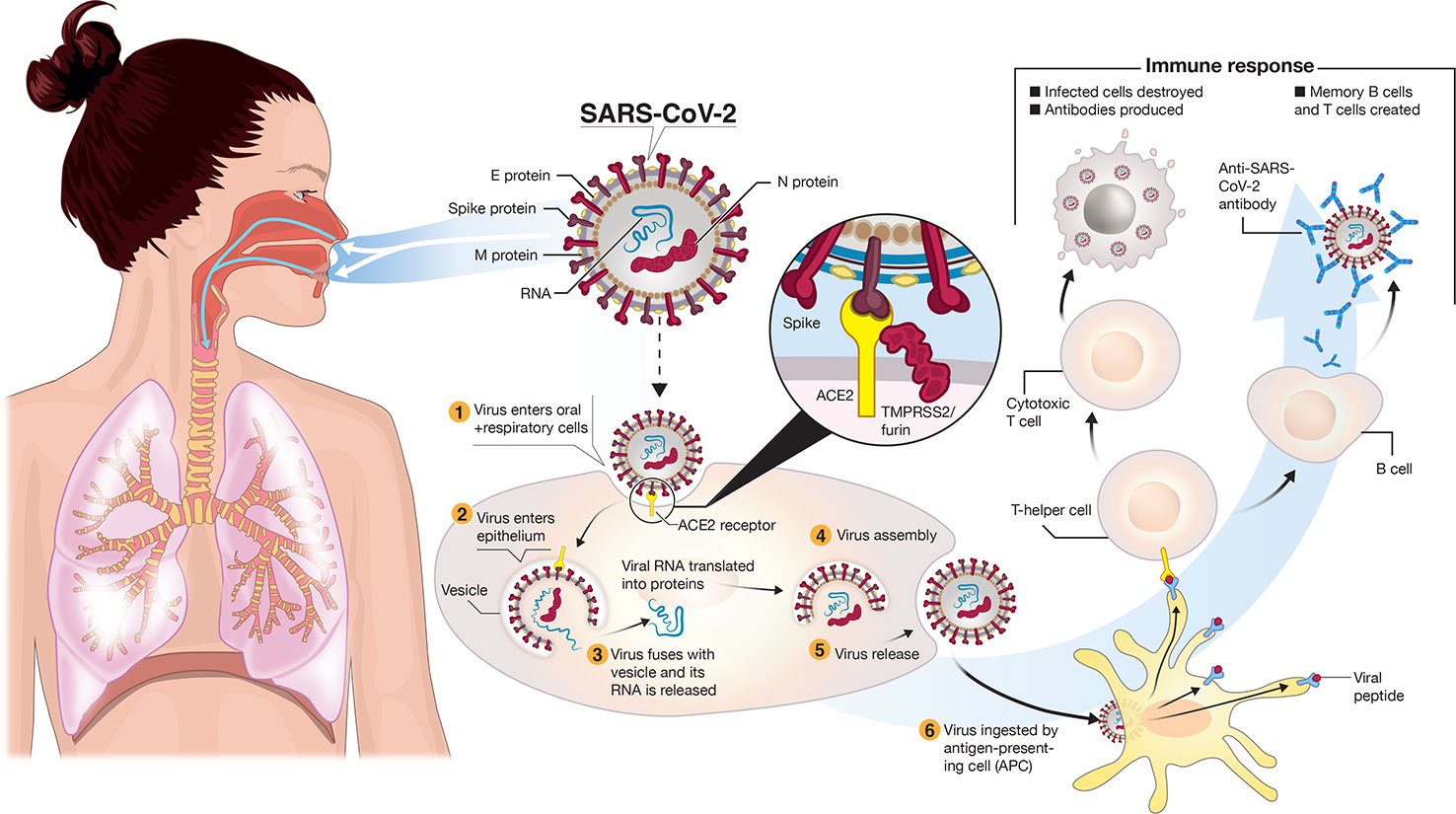


























/posttv-thumbnails-prod.s3.amazonaws.com/thumbnails/5fa9577a46e0fb000112791e/2020-11-09T144422Z_1_OVD3UPZZF_RTRMADC_0_HEALTH-CORONAVIRUS-VACCINES-PFIZER.jpg)