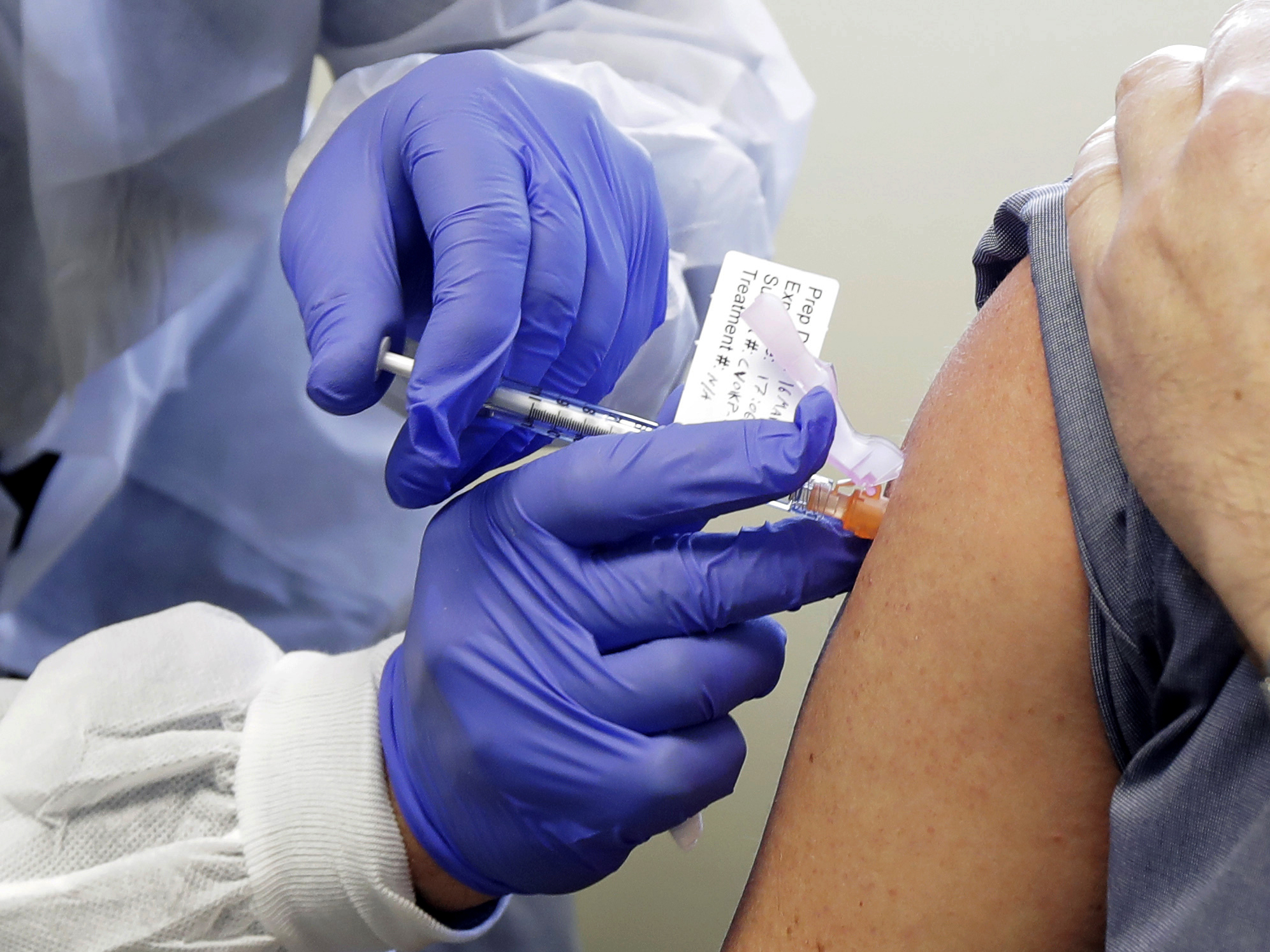
Operation Warp Speed Phase 3 Trials
The new study known as a phase 3 trial is expected to enroll up to 10000 people in the united kingdom.

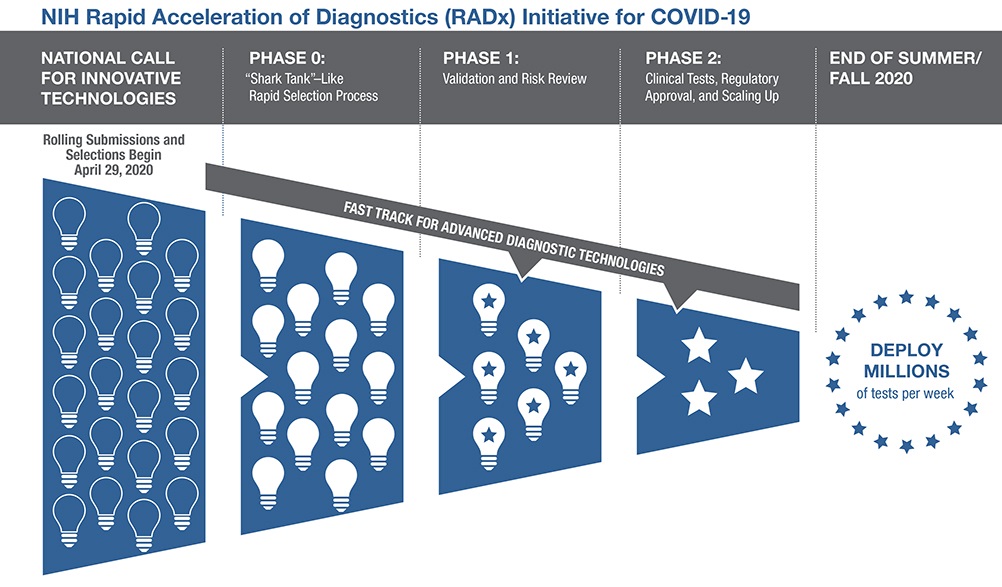
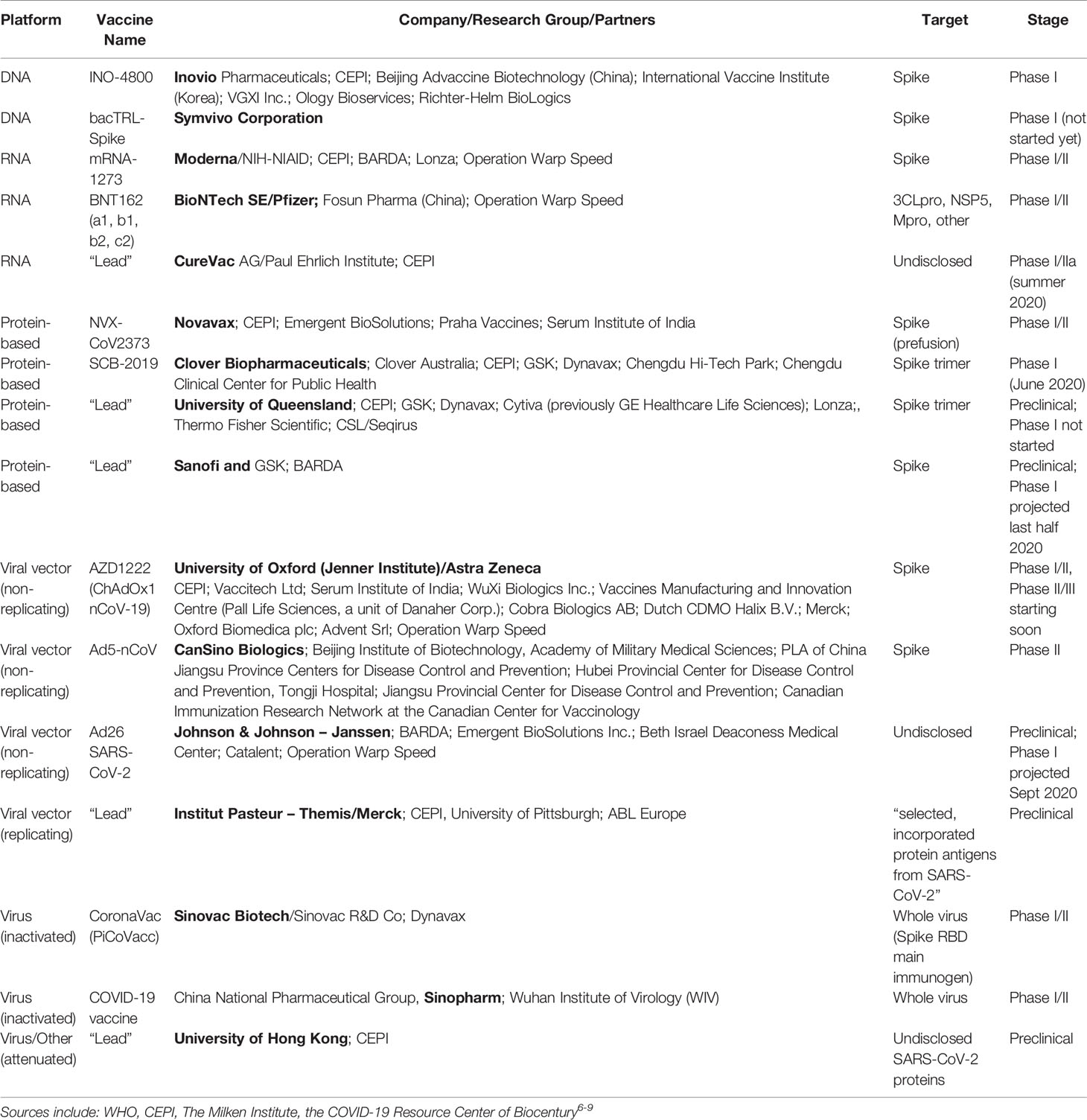
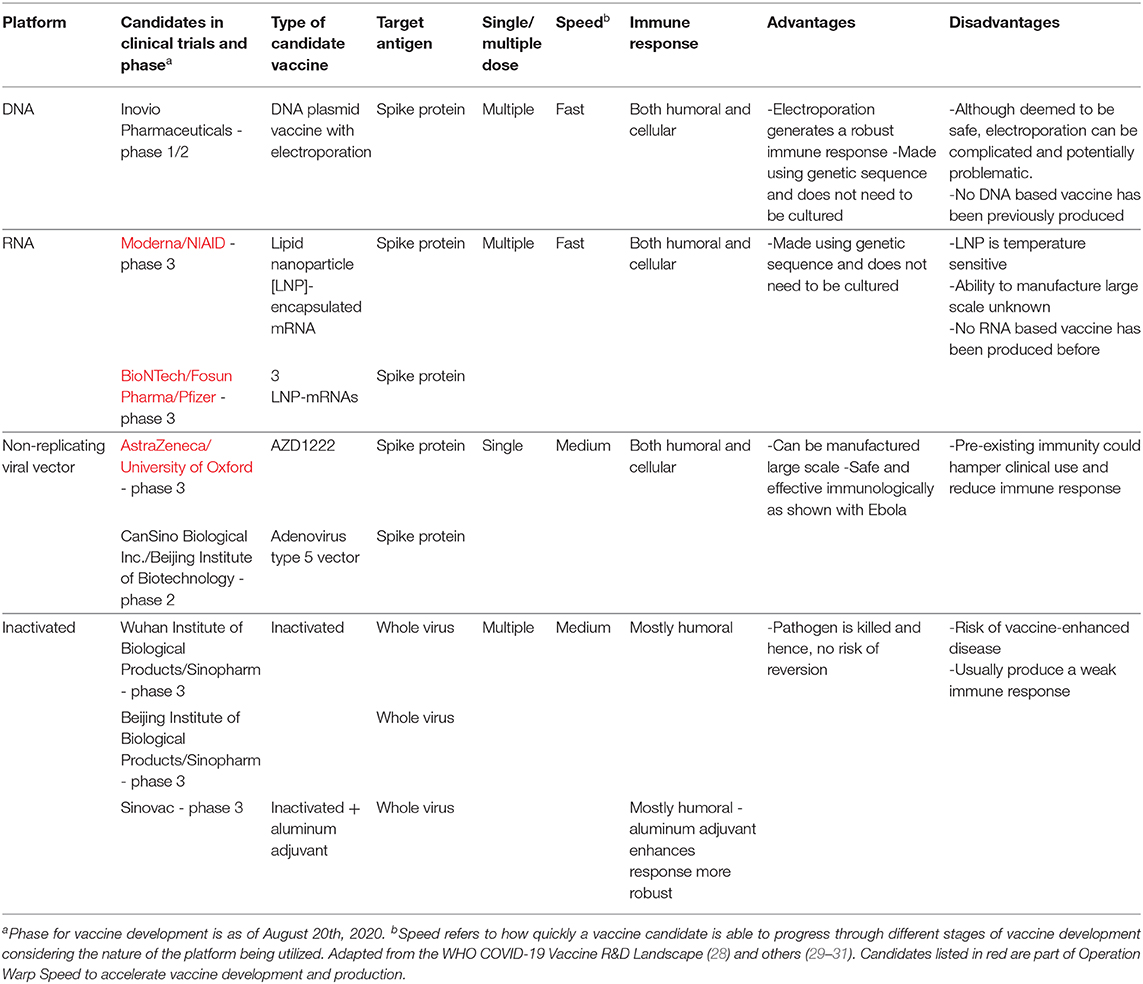
Operation warp speed phase 3 trials. Phase 3 open to accrual projected as of july 29 2020 july 27 2020. As part of the agencys efforts to combat covid 19 the fda issued an emergency use authorization eua for investigational convalescent plasma. Up to 60000 volunteers will be enrolled in the trial at up to nearly 215 clinical research sites in the united states and internationally. Novavax one of the biotech firms that received funding from the trump administrations operation warp speed to produce a coronavirus vaccine announced tuesday it plans to begin phase 3 clinical.
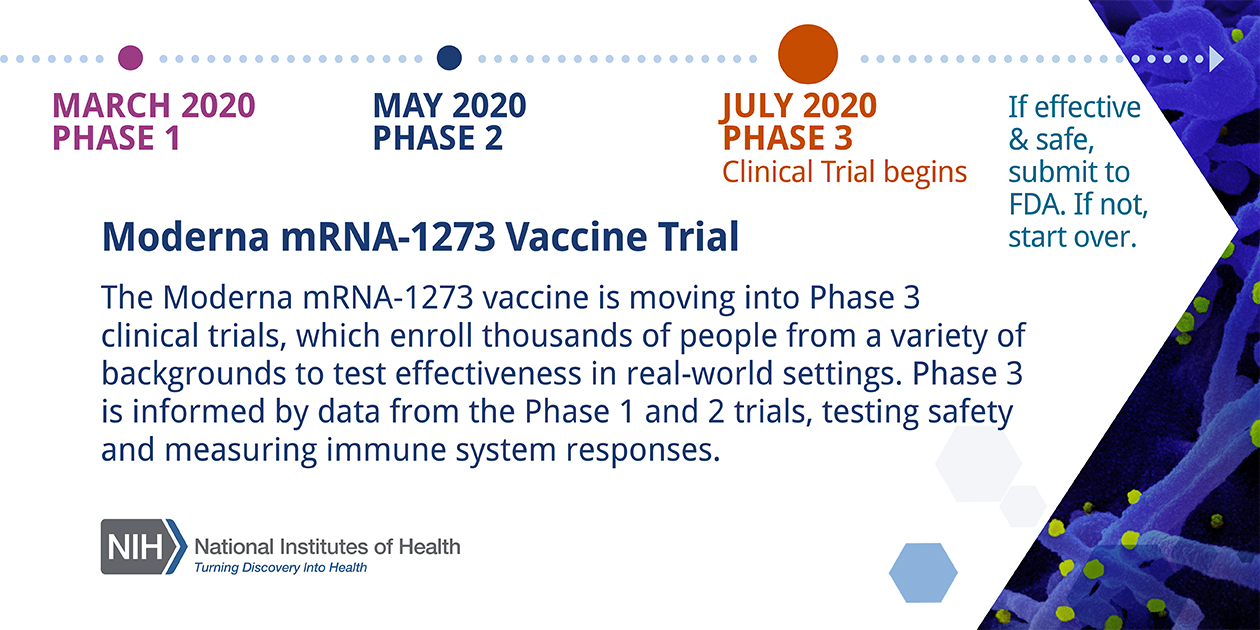
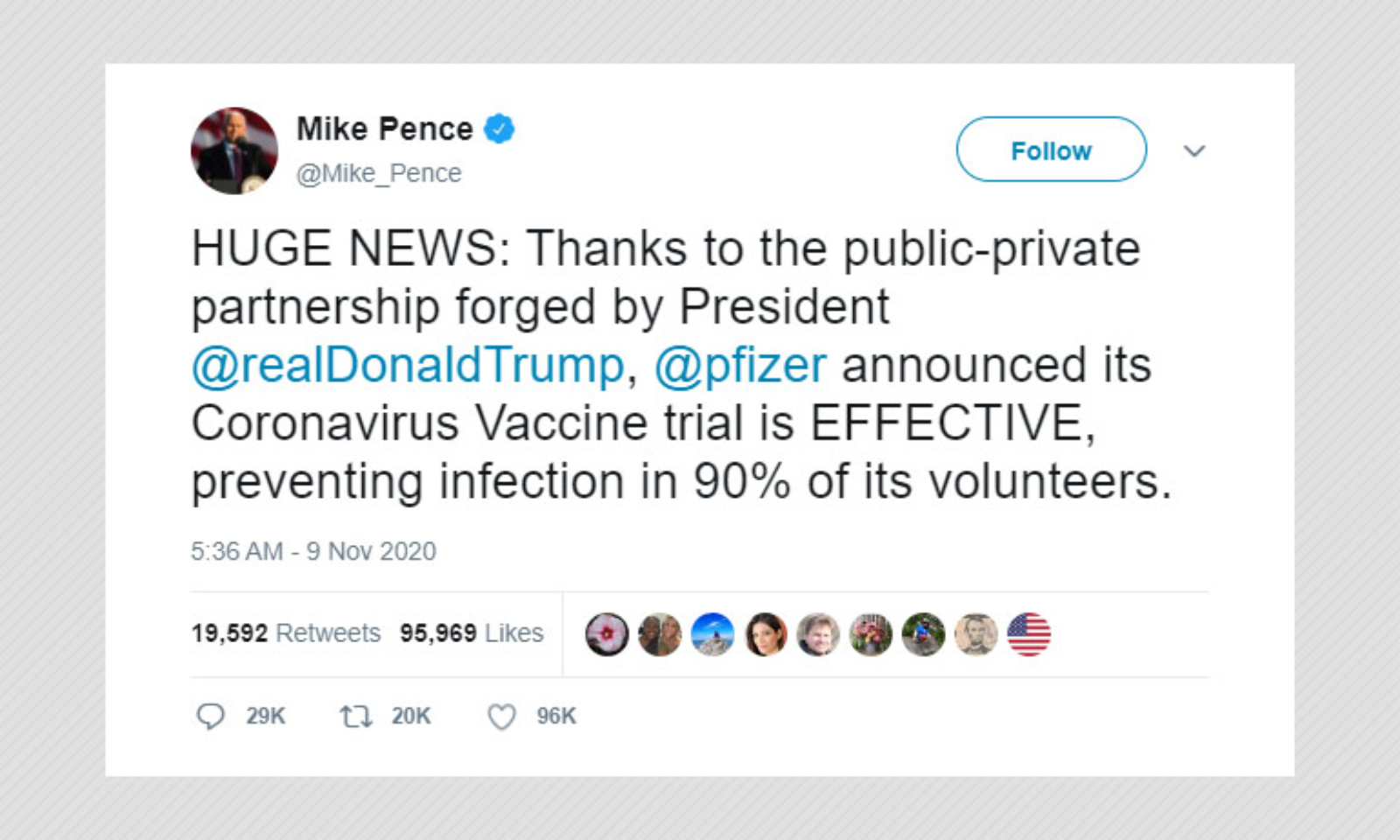
Phase 3 efficacy trials. The phase 3 clinical trial which began july 27 is the first government funded phase 3 clinical trial for a covid 19 vaccine in the united states. The trump administration has set a goal to deliver 300 million doses of a coronavirus vaccine by january through its initiative operation warp speed. Phase 3 vaccine trials are currently underway.
Moderna is one of several pharmaceutical companies partnered with trumps operation warp speed which is intended to produce at least 300 million vaccine doses by january 2021. Moderna has enrolled all 30000 volunteers needed for its late stage trial and expects to have its first interim analysis this month tootwo other companies affiliated with operation warp speed. Half of the volunteers will receive two doses of the experimental vaccine 21 days apart.
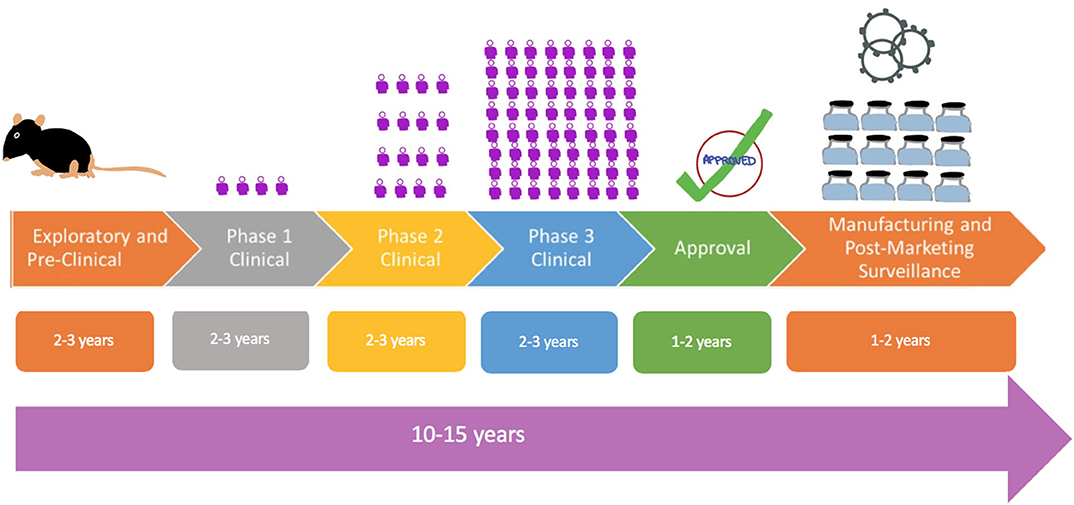


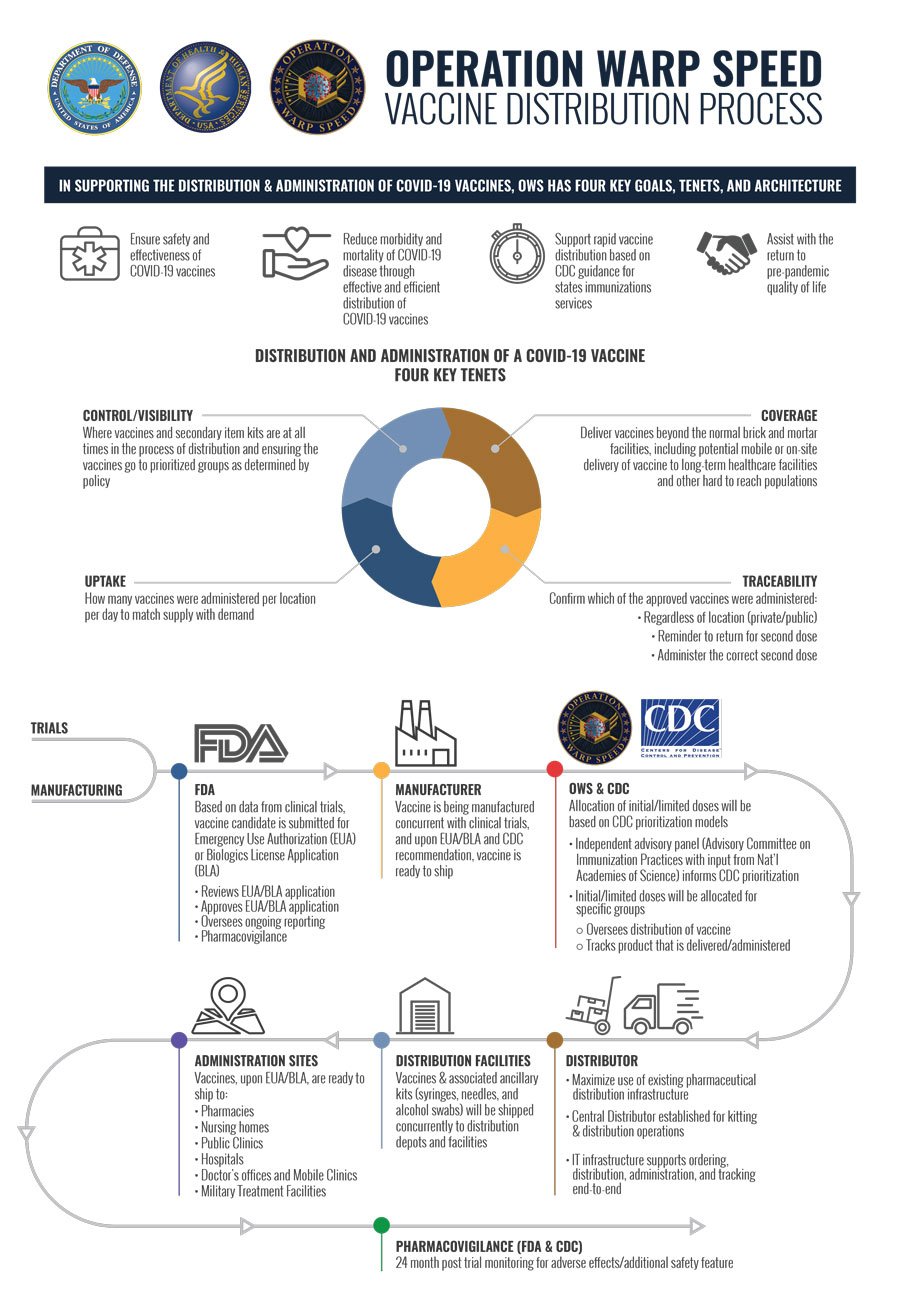


























































/cdn.vox-cdn.com/uploads/chorus_image/image/67596019/GettyImages_1213154990.0.jpg)