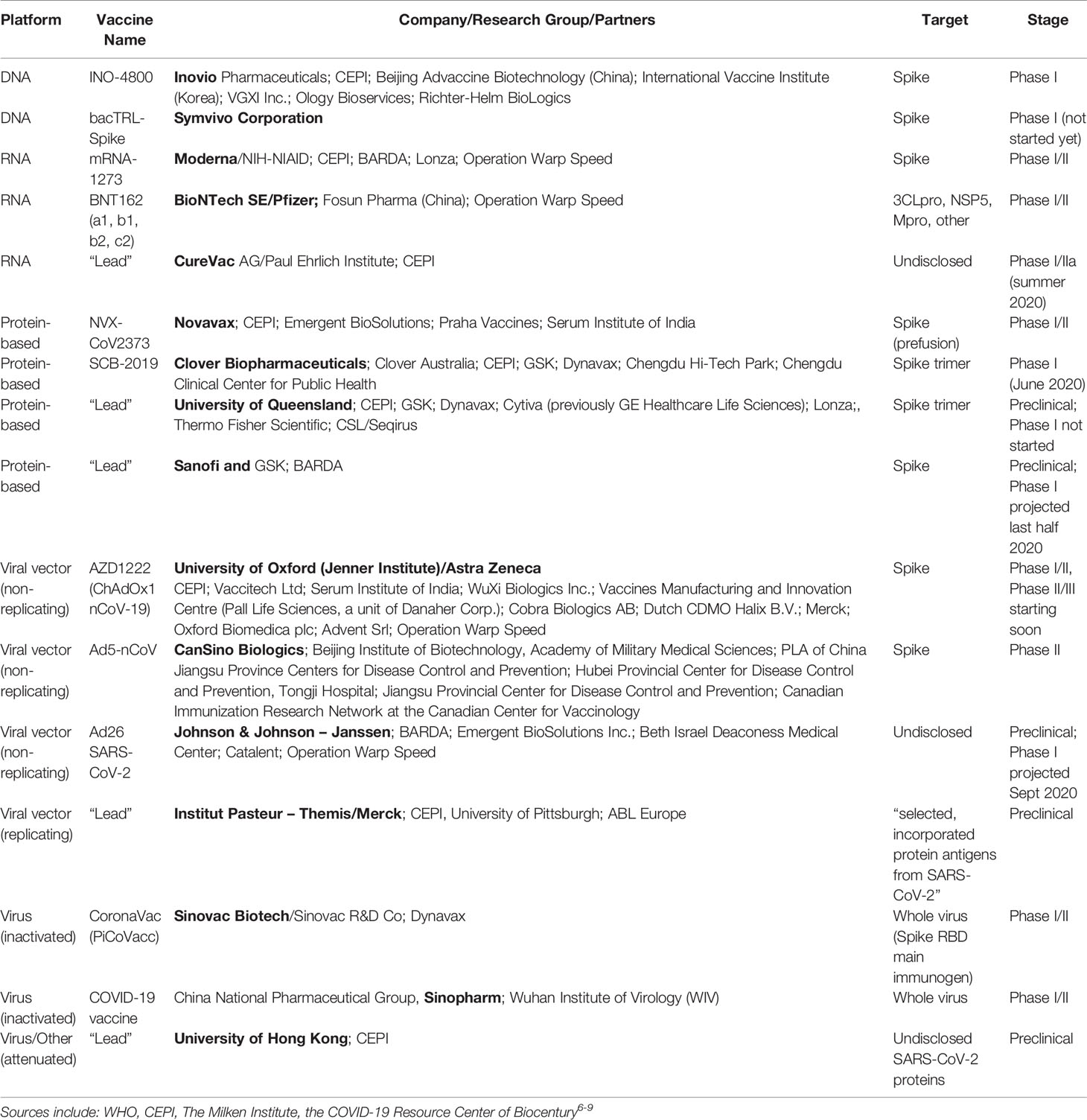
Operation Warp Speed Nhp Study
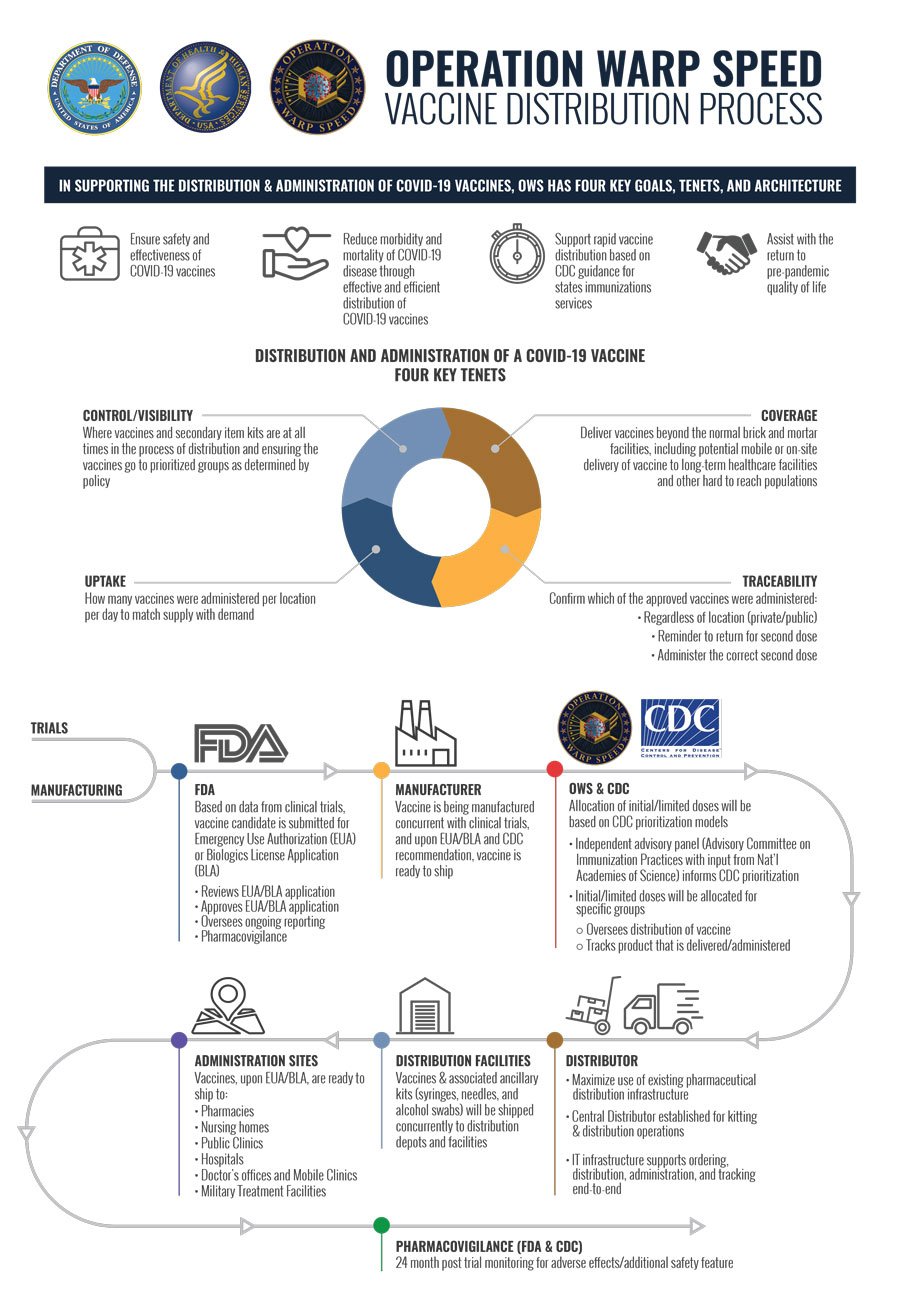
Operation warp speeds goal is to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by january 2021 as part of a broader strategy to accelerate the development manufacturing and distribution of covid 19 vaccines therapeutics and diagnostics collectively known as countermeasures.

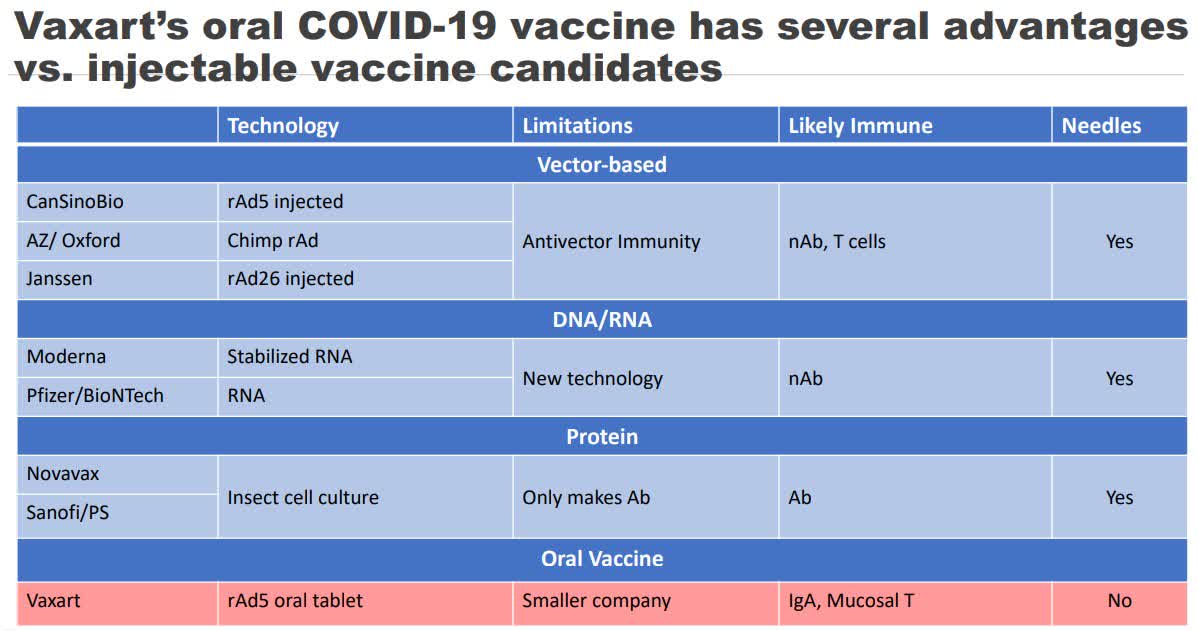
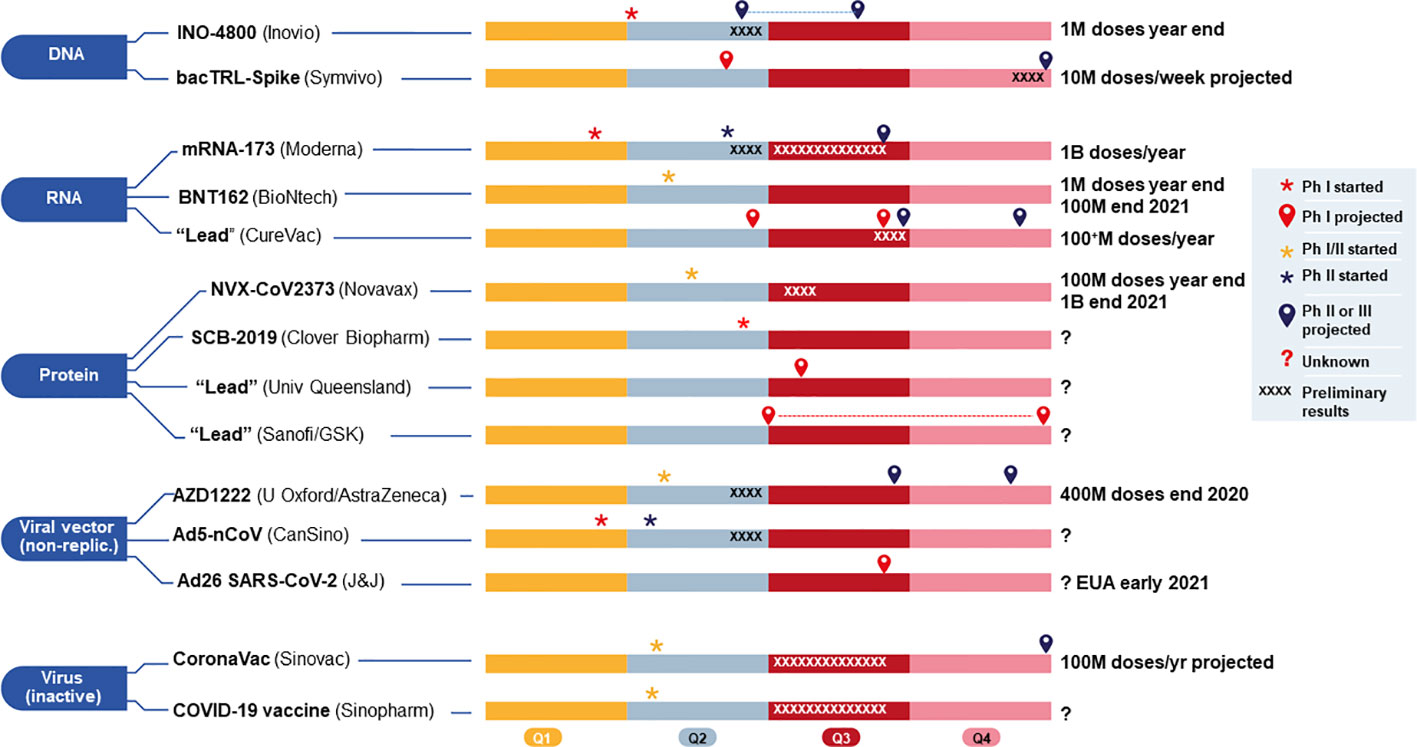
Operation warp speed nhp study. This large scale study definitely has the potential to save lives. South san francisco calif june 26 2020 globe newswire vaxart inc a clinical stage biotechnology company developing oral vaccines that are administered by tablet rather than by injection today announced that its oral covid 19 vaccine has been selected to participate in a non human primate nhp challenge study organized and funded by operation warp speed a new national program aiming to provide substantial quantities of safe effective vaccine for americans by january 2021. Furthermore inovio has expanded its phase 1 trial to add older participants in additional cohorts and plans to initiate a phase 23 efficacy trial this summer upon regulatory concurrence. Governments operation warp speed a new national program aiming to provide substantial quantities of safe effective vaccine for americans by january 2021.
Department of health and human services. The new study will be organized and funded by operation warp speed ows which is a new national program to provide substantial quantities of safe effective vaccine for americans by january 2021. On june 26 2020 vaxart inc stated this study is designed to demonstrate the efficacy of vaxarts oral covid 19 vaccine in humans. Inovio also said that ino 4800 has been selected for a non human primate nhp challenge study organized and funded by operation warp speed the program through which president donald trumps.
Pfizer claimed it was never part of the federal governments operation warp speed which aimed to begin delivery of 300 million doses of an fda authorized safe and effective vaccine for covid 19 by the end of the year as part of a broader strategy to accelerate the development manufacturing and distribution of covid 19 vaccines. In addition ino 4800 has been selected to participate in a non human primate nhp challenge study as part of the us. South san francisco calif june 26 2020 globe newswire vaxart inc a clinical stage biotechnology company developing oral vaccines that are administered by tablet rather than by. Eleven vaccines are currently in late stage trials worldwide with four frontrunners in the us sponsored by the federally funded operation warp speed.



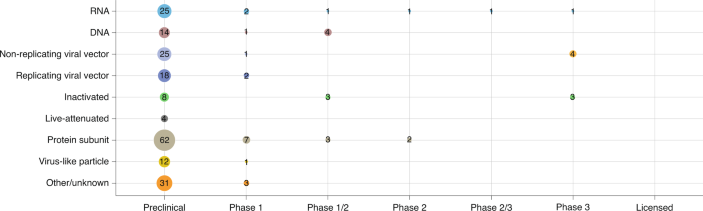
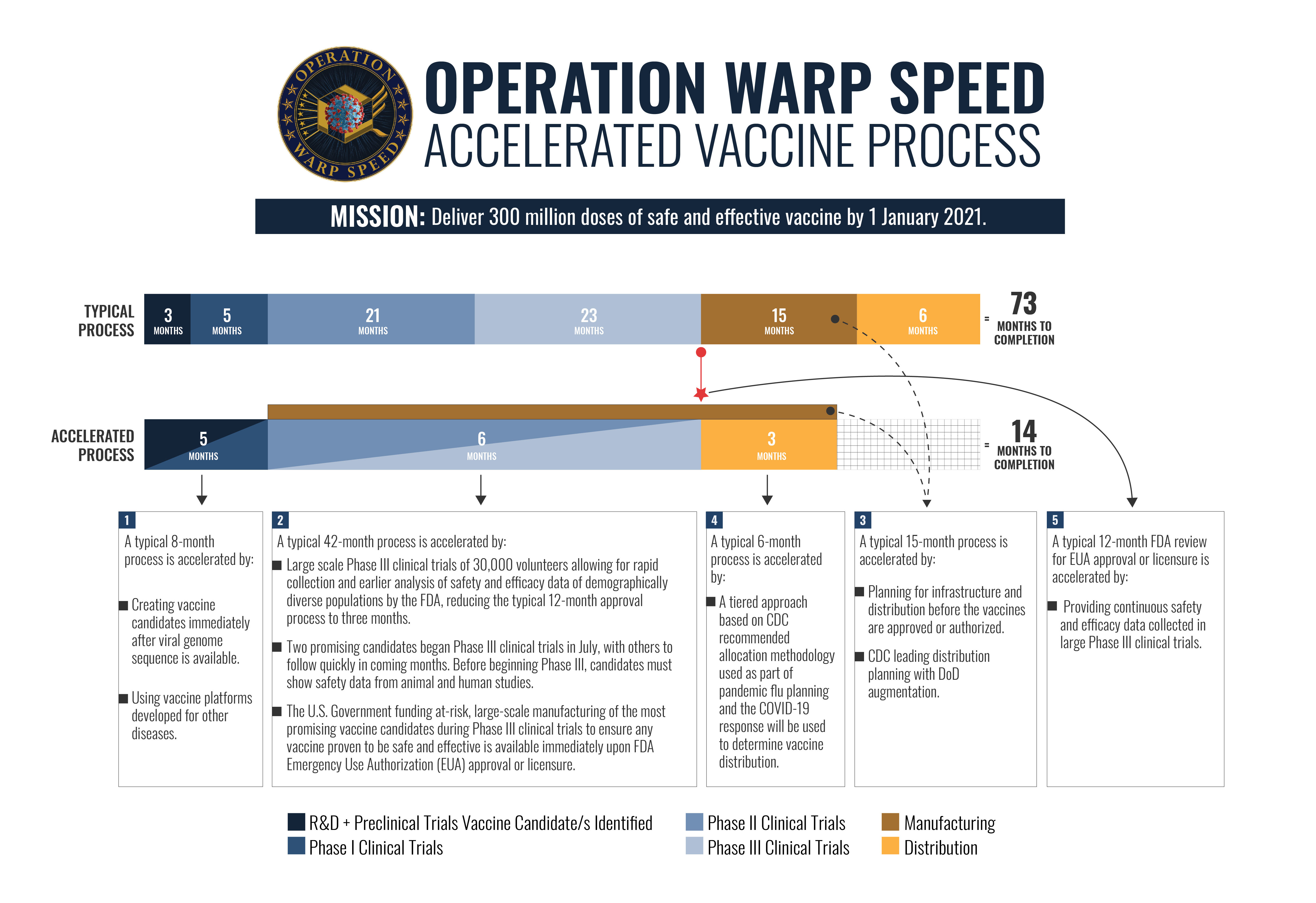




































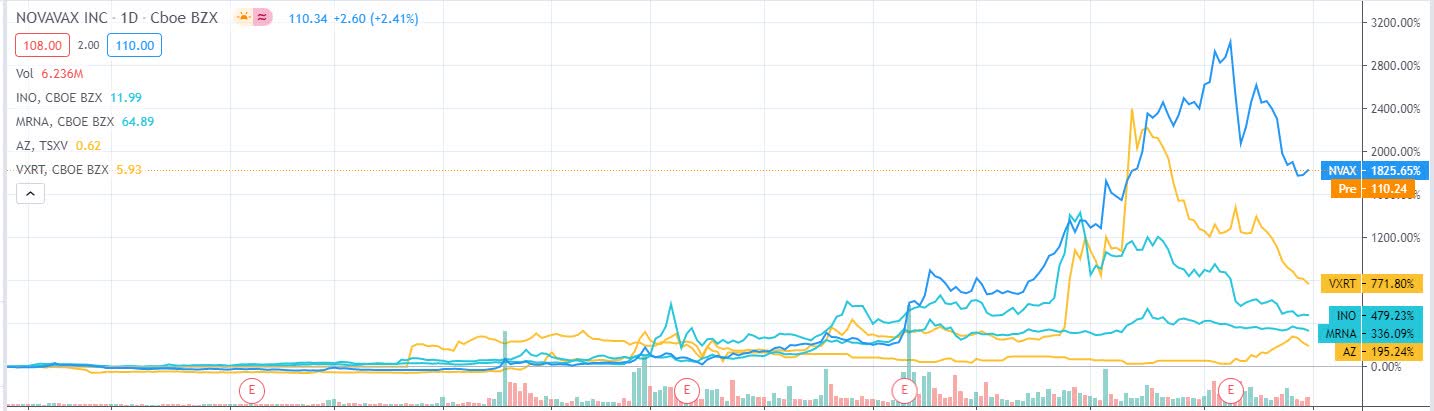



.jpg)