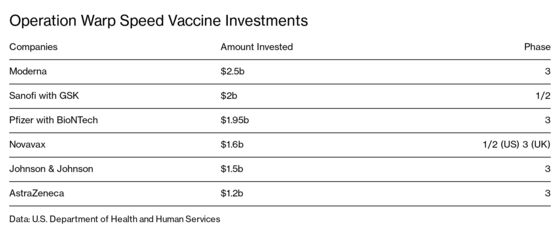
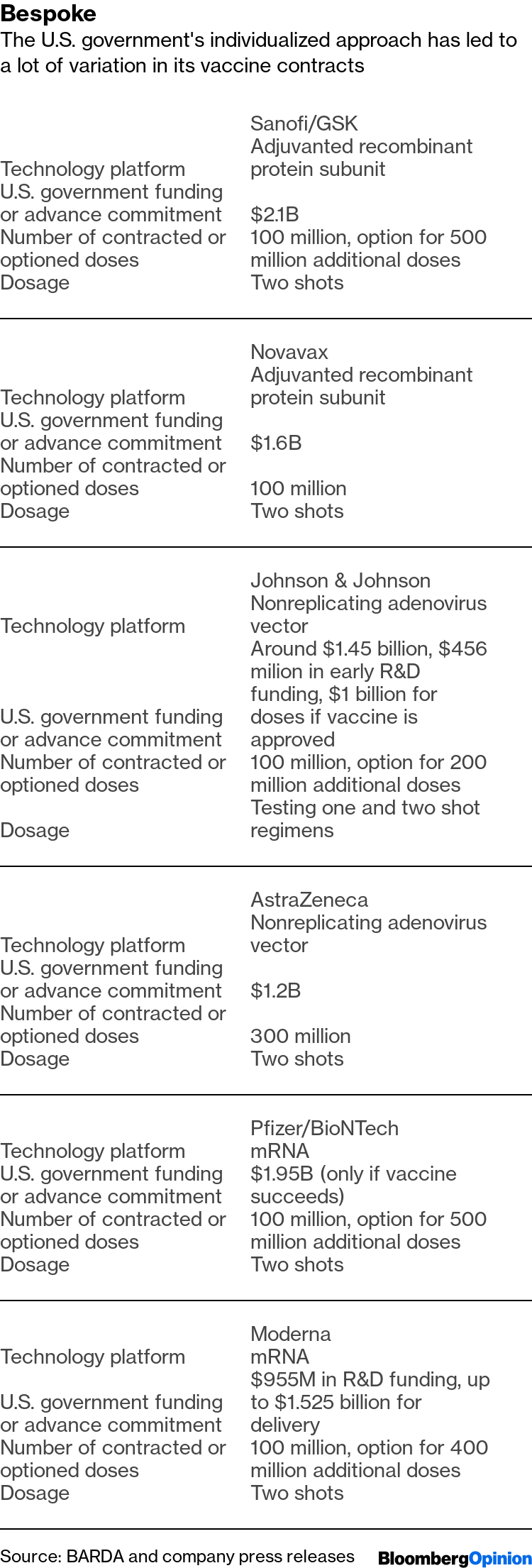
Operation Warp Speed Candidates List
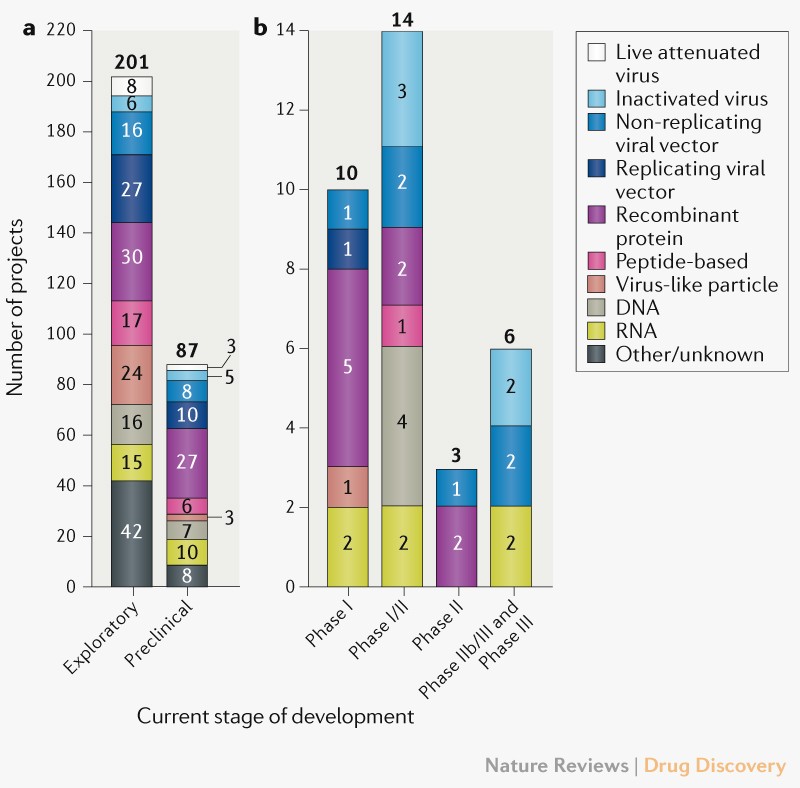
When operation warp speed was initially announced the federal government initially looked about 100 vaccine candidates.

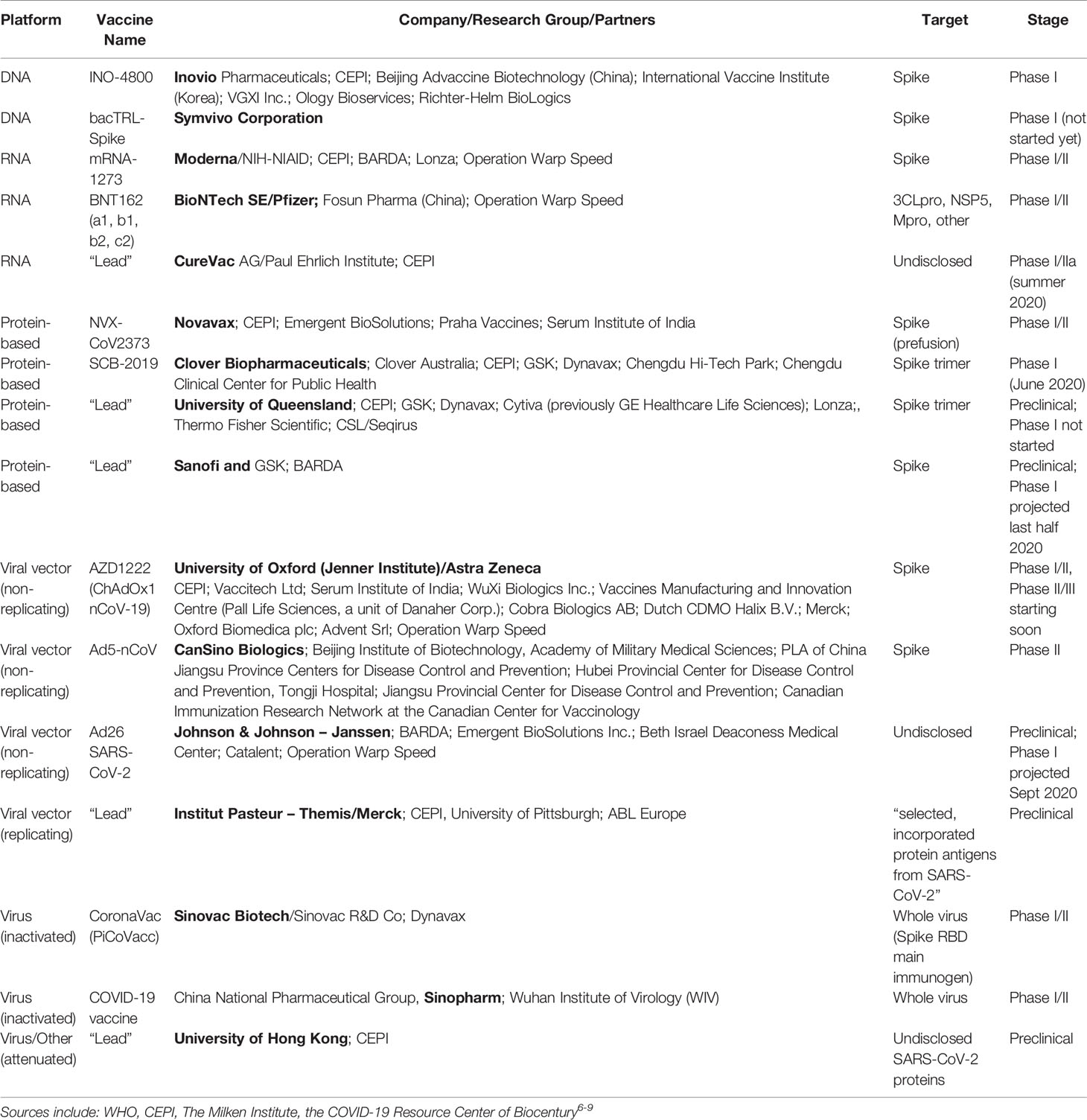
Operation warp speed candidates list. The idea for us is to pick a diversified portfolio of vaccines. Among numerous candidates that vaccine is the furthest along in development entering a phase 2 trial. In an effort to bring an effective covid 19 vaccine to the us market by 2021 the white house launched operation warp speed ows last month and today named five vaccine candidates as the most likely to produce a viable vaccine in a record breaking timeframe. The factors guiding the decisions about which projects to fund is unclear.
Astrazeneca and oxford universitys azd1222 now in clinical trials at multiple uk sites. Operation warp speed will select the most promising countermeasure candidates and provide coordinated government support to support their development. Warp speed has already narrowed its list of vaccine candidates to 14 and plans to push ahead with eight the official says. When operation warp speed was initially announced the federal government initially looked about 100 vaccine candidates.
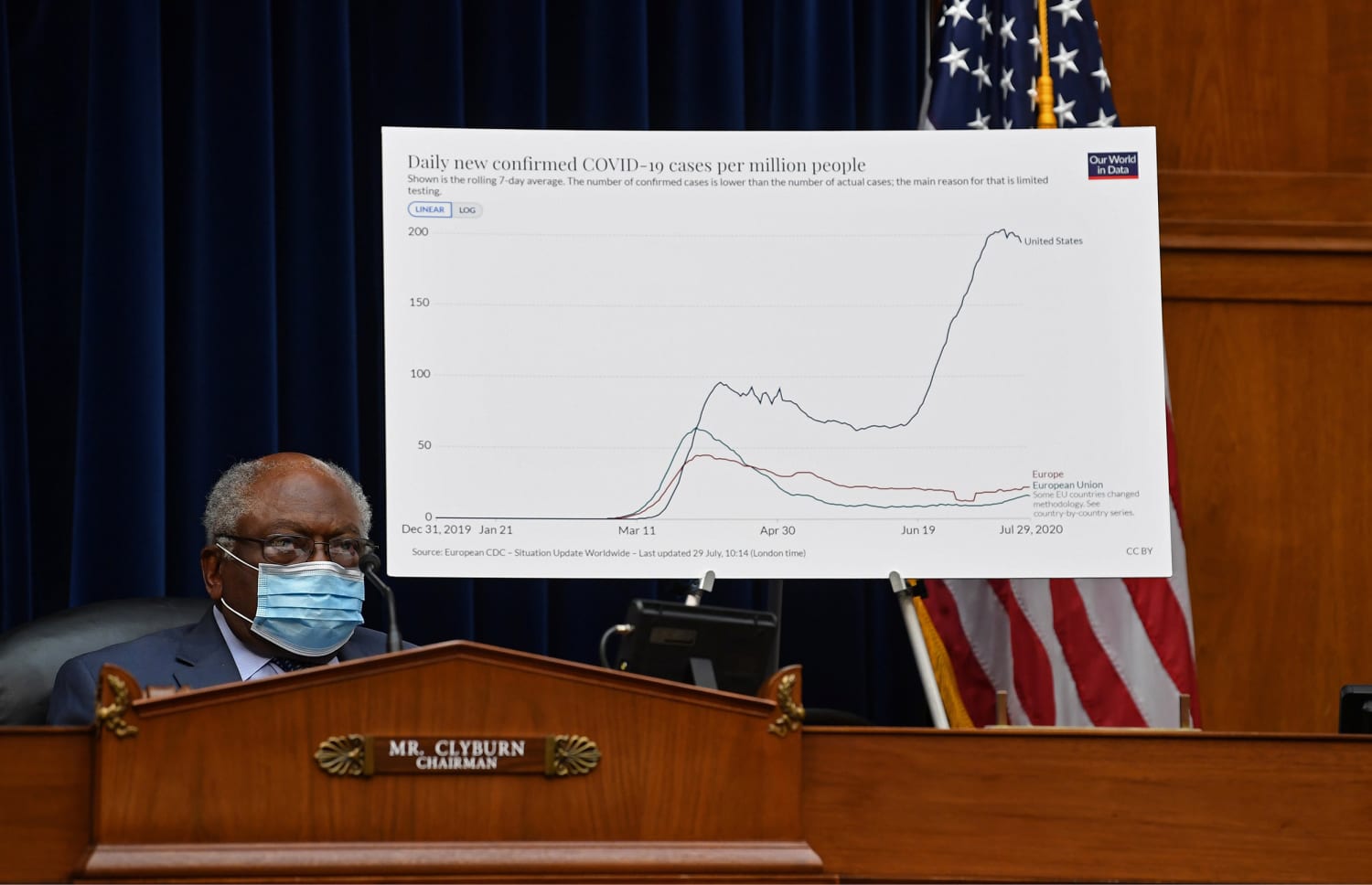
Operation warp speed ows is a publicprivate partnership initiated by the us. Government to facilitate and accelerate the development manufacturing and distribution of covid 19 vaccines therapeutics and diagnosticsoperation warp speed was introduced in early april 2020 after a round table meeting with industry executives at the white house on march 2. Protocols for the demonstration of safety and efficacy will be aligned which will allow the trials to proceed more quickly. As operation warp speed pushes to develop a covid 19 vaccine in record time the number of candidates is being narrowed.
With operation warp speed president trump is trying to push the development of a vaccine. The five vaccines include modernas mrna1273 currently in phase 2 trials. A merck vaccine based on that. The governments goal is to identify the most promising vaccine candidate while it is still under development and provide as much support as possible in order to move it through the clinical and regulatory process in order.











































.jpg)